INNO-LIA® HTLV I/II Score
Finally, a clear answer in HTLV confirmation
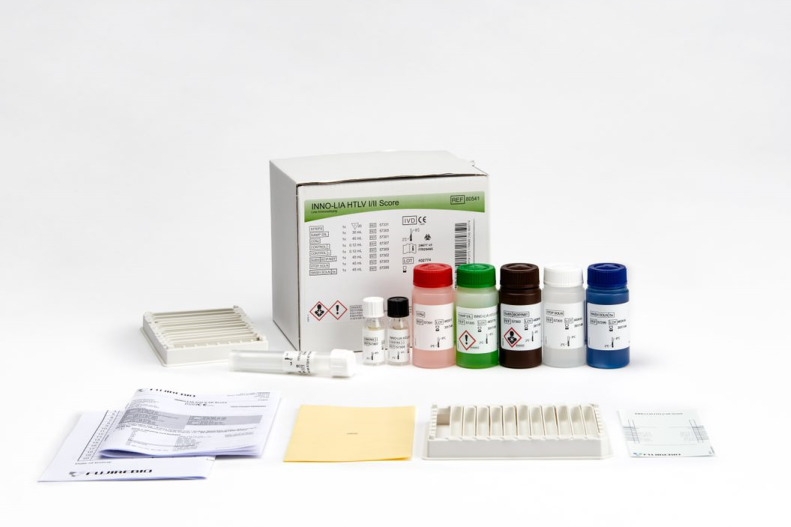
Line immunoassay for the confirmation and discrimination of antibodies to Human T-cell Lymphotrophic Virus type I (HTLV-I) and type II (HTLV-II) in human serum and plasma.
The assay uses well-defined antigens derived from HTLV I and HTLV II immunodominant proteins.
Product number 80541
Click here to navigate
- Contact sales for information
- Details
- Conditions of sale
- Citations
- Documentation
- Product inquiry
- Related products
-
Details
The antigens are either recombinant proteins or synthetic peptides that are purified and fixed on a nylon membrane.
The antigenicity exhibited by these proteins and peptides is either common to HTLV I and HTLV II antibodies or type-specific to one of the two viruses allowing confirmation and discrimination in a single assay.
Two gag bands (p19 I/II, p24 I/II) and two env (gp46 I/II, gp21 I/II) bands are applied as non-type-specific antigens, used to confirm the presence of antibodies against HTLV I/II.
The type-specific antigens (gag p19-I, env gp46-I) specific for HTLV I and (env gp46-II) specific for HTLV II, enable differentiation of HTLV I and HTLV II infections.
Features & Benefits
-
Complete answer in one test: confirmation and discrimination
-
Low follow-up rate
-
Low indeterminate rate
-
Optimal specificity in screened-negative blood donors: 99.7% (304/305)
-
Optimal sensitivity for HTLV I (100%; 218/218) and HTLV II (100%; 112/112)
-
Color-coded reagents
-
Ready-to-use reagents
-
Four control lines to monitor 1) non-specific reactivity, 2) sample addition, 3) and 4) color development steps
-
Fully automated strip processing possible using TENDIGO™, Auto-LIA™ 48, AutoBlot 3000(H) or Roboblot®
-
Objective, automated reading and interpretation of the strips possible using LiRAS® for Infectious Diseases
-
-
Conditions of sale
To read the end user conditions of sale for this product please visit our Resource center.
-
Citations
The BIOZ badges associated with Fujirebio products include peer-reviewed citations derived from scientific studies using Fujirebio products. Please note that the peer-reviewed citations do not reflect the regulatory status of Fujirebio products. Users should refer to the specific product documentation and any (clinical) claims made therein in order to ensure compliant use. For each country or geographic region, users must verify the related regulatory status of the Fujirebio product.
-
Documentation
Browse regulatory documents for this product
Create a (free!) eServices account and start browsing all product documentation right away.
Get access to this section and more
Create a free eServices account now and instantly access multiple digital resources:
- Product documentation
- Selected scientific posters
- Product leaflets
- How-to videos and more…
Multimedia
Watch product videos
Get a (free!) eServices account and benefit from full access to all our online resources.
-
Product inquiry
-
Related products