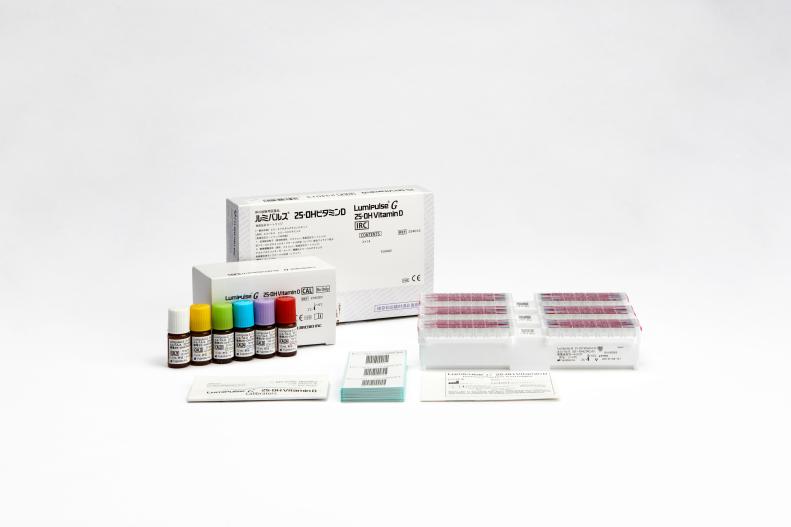
Lumipulse® G 25-OH Vitamin D
New "ray of light" in Vitamin D testing
Lumipulse G 25-OH Vitamin D is an immunoreaction cartridge for in vitro diagnostic (IVD) use with the LUMIPULSE G System for the quantitative determination of 25-hydroxyvitamin D (25-OH-D) in human serum or plasma to be used in the assessment of Vitamin D sufficiency.
CE marked (IVDR)
Lumipulse® G 25-OH Vitamin D Immunoreaction Cartridges
Product number 234013
3 x 14 Tests
Lumipulse® G 25-OH Vitamin D Calibrators
Product number 234020
1 x 6 x 1.5 ml
Please contact your local Fujirebio representative for the availability of this product in your country.