INNO-LiPA® HLA-A Update
Detects all known alleles of HLA-A with one amplification and two strips
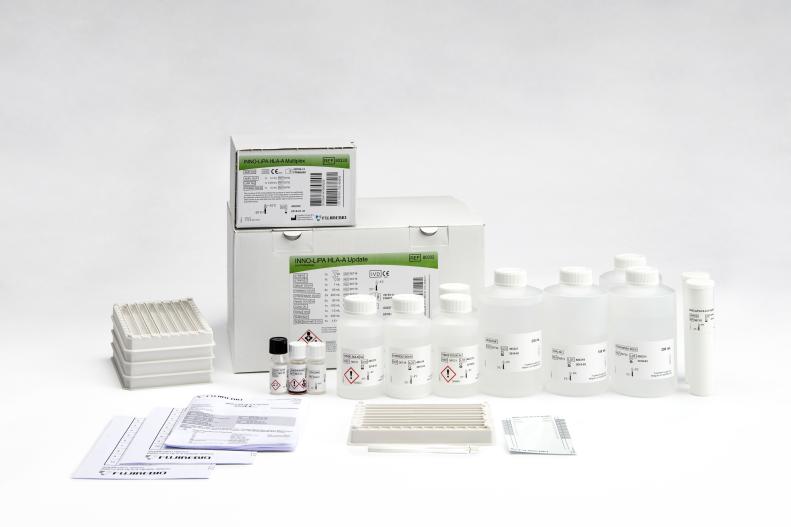
INNO-LiPA HLA-A Update is a line probe assay for the molecular typing of human leukocyte antigen (HLA) A alleles at allele group level (A*01–A*80).
Please note that this product now only is available for sale in Spain and Italy. Contact us for more details.
CE marked
INNO-LiPA® HLA-A Update
Product number 80332
20 Tests
INNO-LiPA® HLA-A Update
Product number 80688
100 Tests
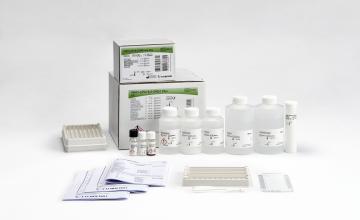
INNO-LiPA® HLA-A Update Multiplex
Product number 80333
20 Tests
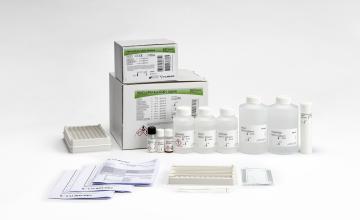
INNO-LiPA® HLA-A Update Multiplex
Product number 80689
100 Tests
Please contact your local Fujirebio representative for the availability of this product in your country.